TB-500 (Thymosin Beta-4)
Thymosin Beta-4 is a 43-amino acid peptide known for its wide range of potential benefits in animal models. Research suggests that it enhances blood vessel growth, regulates wound healing, reduces inflammation, and mitigates oxidative damage in critical areas such as the heart and central nervous system. This peptide plays a crucial role in protecting tissues, facilitating repair, promoting regeneration, and supporting the restructuring of damaged or injured tissues. Additionally, Thymosin Beta-4 is gaining interest in anti-aging research due to its potential role in promoting longevity and overall health.
Product Usage
This product is intended for research purposes only. It is strictly designated for in vitro testing and laboratory experimentation. All information provided on this website is for educational purposes only. Any introduction into humans or animals is strictly prohibited by law. Only licensed, qualified professionals should handle this product. It is not a drug, food, or cosmetic and must not be misbranded, misused, or mislabeled as such.
Introduction
Thymosin Beta-4 (TB-500) is a synthetic version of a naturally occurring 43-amino acid peptide found in various human and animal cells. Research, including a 2010 study published in the Annals of the New York Academy of Sciences, highlights its potential in repairing cardiac muscle following injuries such as myocardial infarction (heart attack). TB-500 has shown promise in cases where stem cell therapy faces limitations, demonstrating the ability to prevent myocardial cell death, stimulate blood vessel growth, and activate healing processes within the heart. These findings suggest that TB-500 could be a groundbreaking therapeutic agent for restoring damaged cardiac muscle post-heart attack. Earlier studies in mice, conducted in 2004, further support its role in promoting cardiomyocyte migration, survival, and myocardial repair.
Structure
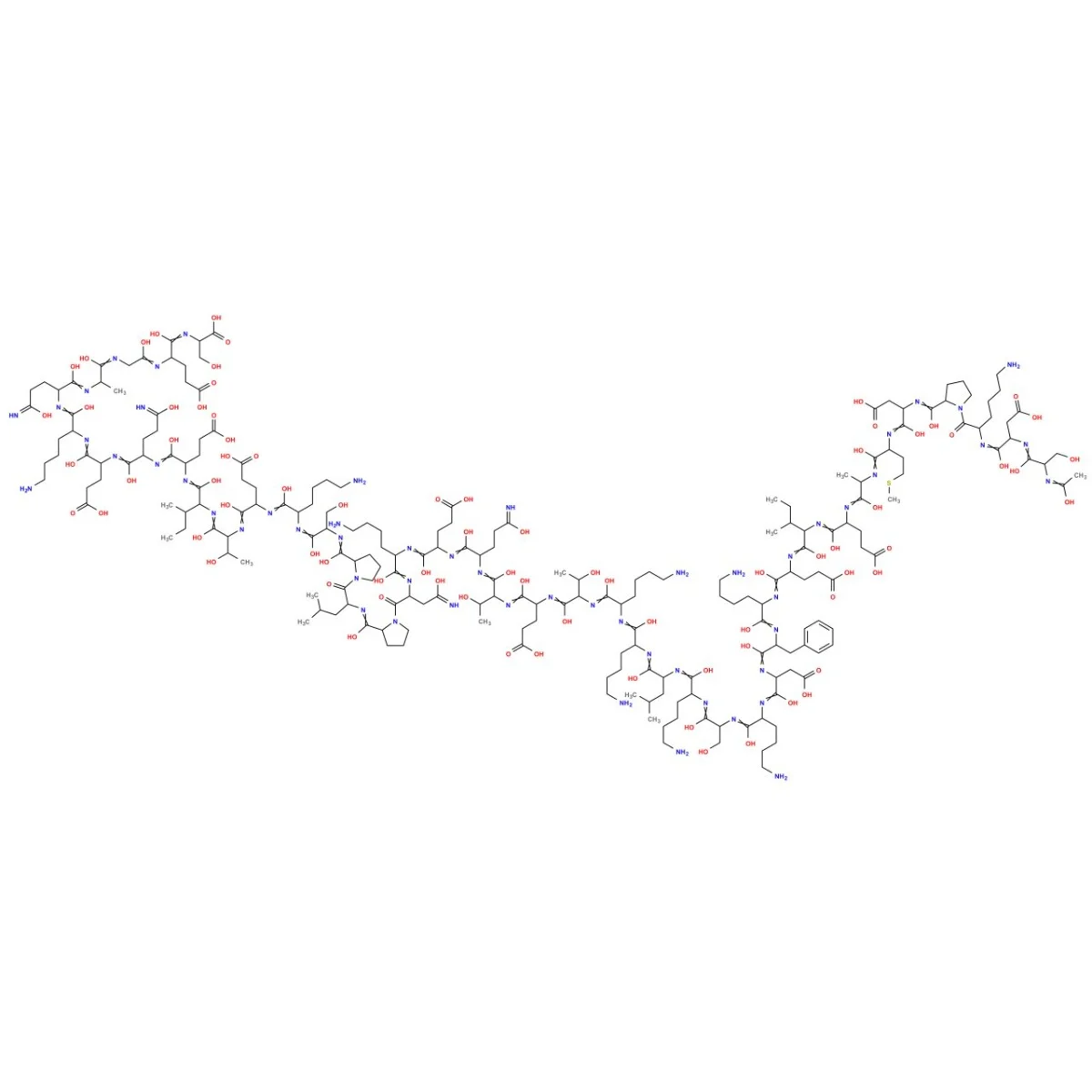
Sequence: Ac-Ser-Asp-Lys-Pro-Asp-Met-Ala-Glu-Ile-Glu-Lys-Phe-Asp-Lys-Ser-Lys-Leu-Lys-Lys-Thr-Glu-Thr-Gln-Glu-Lys-Asn-Pro-Leu-Pro-Ser-Lys-Glu-Thr-Ile-Glu-Gln-Glu-Lys-Gln-Ala-Gly-Glu-Ser
Molecular Formula: C212H350N56O78S
Molecular Weigh: 4963.44 g/mol
PubChem CID: 16132341
Synonyms: Thymosin Beta-4
Research on TB-500
Multiple studies have explored the potential therapeutic benefits of Thymosin Beta-4 (TB-500) across various health conditions:
- Diabetic Eye Surgery: A 2009 study utilizing TB-500 eye drops (0.01% concentration) demonstrated accelerated healing in diabetic patients undergoing eye surgery. Since diabetic individuals, particularly those with diabetic retinopathy, often experience delayed healing, these findings are significant. While no severe side effects were reported, some patients experienced mild symptoms such as headaches, dizziness, and insomnia.
- Cystic Fibrosis and Mucociliary Transport: TB-500, when combined with dornase alfa, was studied in cystic fibrosis patients. The combination therapy significantly reduced sputum cohesivity, enhancing mucociliary transport and improving the clearance of mucus through coughing.
- Liver Disease: Research into TB-500’s effects on chronic hepatitis B and nonalcoholic fatty liver disease (NAFLD) suggested a potential role in reducing inflammation and fibrosis. Although it did not directly impact hepatitis B virus levels or liver function markers, its association with improved liver tissue health warrants further investigation.
- Safety and Tolerance: A 2010 study evaluating TB-500’s safety in humans found that intravenous doses ranging from 42 mg to 1260 mg daily over 14 days did not result in treatment-related adverse effects or dose-dependent toxicity.
- Immune Response and Respiratory Viruses: A study on healthy volunteers exposed to rhinovirus (common cold virus) showed increased levels of serum cortisol, thymosin alpha-1, and TB-500 by the fifth day post-exposure. These changes correlated with heightened immune cell activity, suggesting a role for TB-500 in modulating immune responses to respiratory infections.
- Kidney Disease: In mice, lower natural TB-500 levels were linked to accelerated kidney disease progression. This finding suggests that TB-500 may have a protective role in kidney health.
- Skin and Wound Healing: TB-500 has demonstrated effectiveness in promoting skin repair across various conditions, including burns, diabetic ulcers, and pressure ulcers. It enhances angiogenesis, reduces inflammation, and increases platelet aggregation at wound sites, accelerating the healing process.
- Meta-Analysis Findings: A 2015 meta-analysis of TB-500 research highlighted its potential in diverse medical applications, including tissue regeneration, heart muscle repair post-myocardial infarction, neuroprotection following stroke and brain trauma, improvements in kidney and liver diseases, spinal cord and bone healing, and mitigation of aging-related decline and viral infections.
- Fracture Healing: In a study on mice, TB-500 treatment significantly improved the strength and stiffness of healed fractures compared to untreated controls. Imaging confirmed enhanced bone repair at injury sites.
- Spinal Cord Injury: A study involving rats with spinal cord injuries found that TB-500 administration led to improvements in locomotor function and behavior. It also reduced inflammatory cytokine levels, minimized scar tissue formation, and increased myelin protein levels, indicating potential benefits for spinal cord injury recovery.
- Traumatic Brain Injury (TBI): In rat models of TBI, TB-500 exhibited neuroprotective and neurorestorative effects, including increased blood flow, the generation of new brain cells, and enhanced neural connectivity.
- Multiple Sclerosis (MS): In rat models of MS, TB-500 administration promoted oligodendrocyte generation, reduced axonal damage, and supported axonal remyelination. These effects correlated with functional improvement, suggesting potential therapeutic applications in MS management.
These studies collectively highlight TB-500’s potential across various medical conditions. However, further clinical trials in humans are necessary to confirm its safety and efficacy in therapeutic applications.
DISCLAIMER: FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY
All articles and product details provided on this website are strictly for informational and educational purposes.
The product information presented here applies solely to in-vitro studies—experiments conducted outside of living organisms, such as in laboratory settings. These products are not classified as medicines or drugs and have not been approved by the FDA for the prevention, treatment, or cure of any medical conditions, ailments, or diseases.

