Tesamorelin
Tesamorelin is a synthetic analog of growth hormone-releasing hormone (GHRH) primarily used to treat HIV-associated lipodystrophy, a condition characterized by abnormal fat distribution in individuals with HIV. Additionally, ongoing research explores its potential benefits in promoting peripheral nerve health, slowing the progression of mild cognitive impairment, and reducing fat mass.
Product Usage
This product is intended for research purposes only. It is strictly designated for in vitro testing and laboratory experimentation. All information provided on this website is for educational purposes only. Any introduction into humans or animals is strictly prohibited by law. Only licensed, qualified professionals should handle this product. It is not a drug, food, or cosmetic and must not be misbranded, misused, or mislabeled as such.
Introduction
Tesamorelin is a synthetic analog of growth hormone-releasing hormone (GHRH), modified with a trans-3-hexanoic acid group to enhance its stability and efficacy. Developed by Theratechnologies in Canada, it received FDA approval in 2010 for the treatment of HIV-associated lipodystrophy. Beyond its approved use, research is investigating its potential role in promoting peripheral nerve regeneration and as a possible treatment for mild cognitive impairment (MCI), a condition that may precede dementia.
Structure
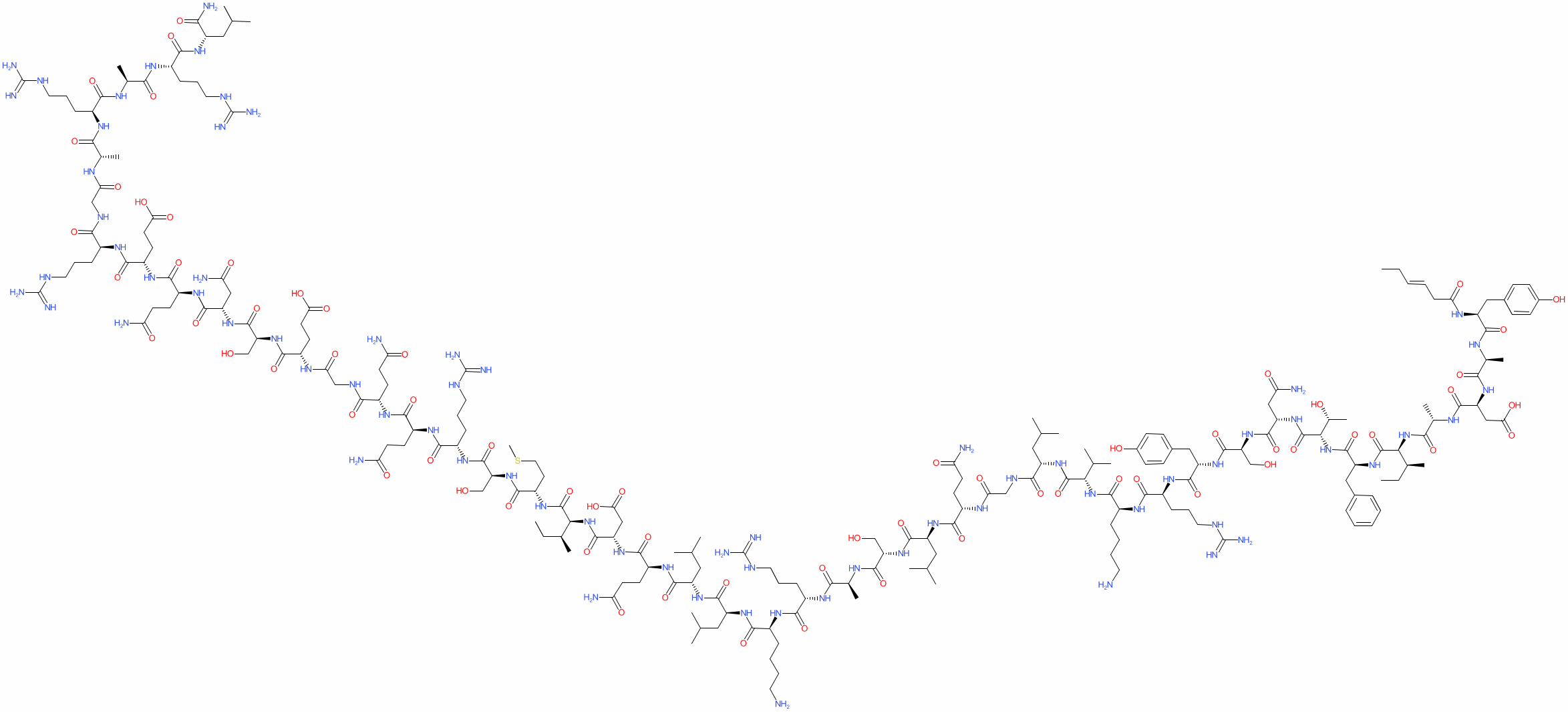
Sequence: Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-Gln-Gln-Gly-Glu-Ser-Asn-Gln-Glu-Arg-Gly-Ala-Arg-Ala-Arg-Leu
Molecular Formula: C223H370N72O69S
Molecular Weigh: 5195.908 g/mol
PubChem CID: 44147413
CAS Number: 901758-09-6
Research on Tesamorelin
Tesamorelin: A Growth Hormone-Releasing Hormone (GHRH) Analogue
Tesamorelin is a synthetic analogue of growth hormone-releasing hormone (GHRH) that shares similarities with other GHRH analogues, such as sermorelin, GRF (1-29), and CJC-1295. The addition of a trans-3-hexanoic acid group enhances its stability in human plasma, extending its half-life while maintaining the physiological effects of GHRH. Unlike other compounds that disrupt natural growth hormone (GH) secretion, tesamorelin preserves the body’s pulsatile GH release, minimizing side effects.
Tesamorelin for HIV-Associated Lipodystrophy
Tesamorelin is primarily used to treat HIV-associated lipodystrophy, a condition linked to both HIV infection and antiretroviral therapy. This disorder causes excessive fat accumulation, particularly in the abdominal region. Although the exact cause is not fully understood, protease inhibitors used in HIV treatment are believed to contribute significantly.
Before tesamorelin’s approval, treatment options for lipodystrophy were limited to lifestyle modifications, such as diet and exercise, or invasive surgical procedures with mixed results. In 2010, the FDA approved tesamorelin as the first effective therapy for HIV-associated lipodystrophy, demonstrating a nearly 20% reduction in adiposity. Research suggests that tesamorelin is approximately four times more effective than all other available treatments combined.
Tesamorelin and Cardiovascular Health
HIV-positive individuals are at a higher risk of developing cardiovascular disease (CVD) due to abnormal fat distribution and the metabolic effects of antiretroviral therapy. Managing cardiovascular risk factors is critical for long-term health in HIV patients.
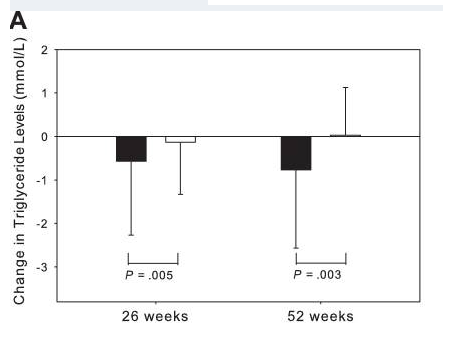
Studies show that tesamorelin not only reduces lipodystrophy but also helps lower triglyceride levels, total cholesterol, and non-HDL-C levels. Research indicates that a 15% reduction in visceral adipose tissue from tesamorelin treatment is associated with a 50 mg decrease in triglyceride levels, highlighting its potential cardiovascular benefits.
Reducing Inflammation and Cardiovascular Risk
Ectopic fat accumulation—such as visceral, liver, and epicardial fat—is linked to inflammation, a key contributor to cardiovascular disease. By reducing ectopic fat deposits, tesamorelin helps mitigate inflammation, lowering the risk of CVD and improving overall metabolic health.
Growth Hormone Deficiency in HIV Patients
Highly active antiretroviral therapy (HAART) has been associated with various endocrine and metabolic complications, including growth hormone deficiency. Studies suggest that up to one-third of HIV patients undergoing HAART experience reduced GH production due to pituitary gland alterations. This deficiency may contribute to the high prevalence of lipodystrophy in HIV-positive individuals.
Tesamorelin offers a safer and more effective alternative to direct GH supplementation, helping restore healthy GH levels while avoiding the risks associated with exogenous GH administration.
Potential for Treating Peripheral Nerve Damage
Peripheral nerve injuries, often caused by trauma, diabetes, or surgical complications, can result in significant motor and sensory impairments. Due to the complexity of nerve regeneration, treatment options remain limited. Emerging research suggests that therapies targeting GH pathways may enhance nerve repair and improve recovery outcomes.
Tesamorelin has shown potential in promoting nerve regeneration, making it a promising candidate for further investigation in treating peripheral nerve damage. Its existing FDA approval provides a strong foundation for future clinical applications.
Tesamorelin’s Role in Cognitive Health
Growing evidence suggests that GHRH analogues, including tesamorelin, may support cognitive function in individuals with early-stage dementia. A randomized, double-blind, placebo-controlled study at the University of Washington School of Medicine examined tesamorelin’s effects over a 20-week period. The findings suggest that tesamorelin may influence cognitive function by increasing gamma-aminobutyric acid (GABA) levels while reducing myo-inositol (MI) concentrations in the brain.
These results highlight tesamorelin’s potential as a therapeutic option for mild cognitive impairment (MCI) and dementia, opening new avenues for research into neurodegenerative disease prevention and treatment.
Conclusion
Tesamorelin offers multiple benefits beyond its FDA-approved use for HIV-associated lipodystrophy. It helps reduce inflammation, supports cardiovascular health, and maintains balanced GH levels in HIV patients. Additionally, its potential role in nerve regeneration and cognitive enhancement makes it an exciting subject for ongoing research, with the possibility of broader medical applications in the future.
DISCLAIMER: FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY
All articles and product details provided on this website are strictly for informational and educational purposes.
The product information presented here applies solely to in-vitro studies—experiments conducted outside of living organisms, such as in laboratory settings. These products are not classified as medicines or drugs and have not been approved by the FDA for the prevention, treatment, or cure of any medical conditions, ailments, or diseases.
Furthermore, the use of these products in humans or animals is strictly prohibited by law.

